Nitrogen and phosphorus are N-type dopants for diamond. 35 Br Bromine 79904 2 8 18 7.

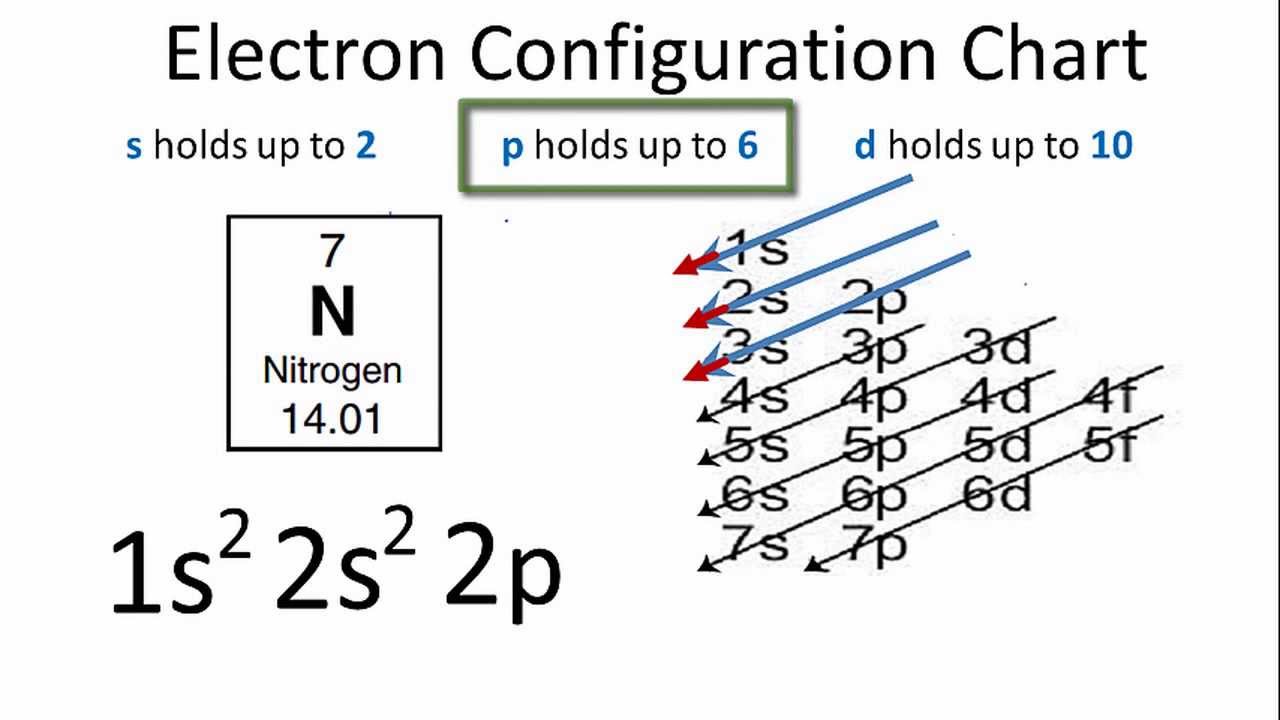
See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Atom Model
In the general case the upward energy band leads to hole accumulation on the surface while the electrons.

Arsenic number of electrons.
The primary use of arsenic is in alloys of lead for example in car.
These group elements are also called chalcogens.
To make the n-type semiconductor pentavalent impurities like phosphorus or arsenic are added.
This Valence Electrons chart table gives the Valence Electrons of all the elements of periodic table.
These group elements are also called pnictogens.
Phosphorus arsenic and antimony are used with silicon.
The total number of electrons present in the valence shell of an atom are called valence electrons.
34 Se Selenium 78971 2 8 18 6.
All these elements have a valency of 2.
The numberletter chunk is the name of the electron orbital and the raised number is the number of electrons in that orbital thats it.
The four electrons are used by the semiconductor atom in forming bonds with its neighbouring atoms.
Members of the group including of course nitrogen along with arsenic phosphorous antimony and bismuth are particularly stable in compounds because they tend to form double or triple covalent bonds.
The Sun is made up of mostly hydrogen.
Arsenic is a chemical element with the symbol As and atomic number 33.
However both carriers do not necessarily move with the same velocity with the application of an external field.
37 Rb Rubidium 84468 2 8 18 8 1.
Hence number of free electrons number of holes in n-type semiconductor That is why free electrons are called majority carriers and holes are called minority carriers in the n-type semiconductorAs the negatively charged electrons mainly involve in charge transferring through this semiconductor it is referred to as negative type or n-type semiconductor.
Atoms are composed of 3 kinds of small particles.
And as there must be an equal number of electrons and protons in an element there must also be 10 Electrons in the element.
The element atomic number and name are listed in the upper left.
All these elements have a valency of 3.
Energy Diagram of n-Type Semiconductor The Energy level diagram of the n-type semiconductor is shown in the figure below.
Number of Electrons - How to find the Number of Electrons.
Arsenic 74921 2 8 18 5.
Click here to buy a book photographic periodic table poster card deck or 3D print based on the images you see here.
Remember a neutral atom contains the same number of protons and electrons.
Click on Element Atomic Number Element Symbol Element Name and Element Valence Electrons.
The upper right side shows the number of electrons in a neutral atom.
Arsenic atomic number 33 lies in between phosphorus and antimony in group 15 the so called Nitrogen group of the periodic table.
41 Nb Niobium 92906 2 8 18 12 2.
39 Y Yttrium 88906 2 8 18 9 2.
The number of protons the number of neutrons and the number of electrons an atom has determines what the element it is.
Nitrogen phosphorus arsenic and antimony.
Fourteen days after dosing with gallium arsenide 907 or - 354 of the arsenic and 994 or - 387 of the gallium was eliminated in the feces in the 1000 mgkg group.
The number of protons in atom of gold is therefore 10.
43 Tc Technetium 98.
36 Kr Krypton 83768 2 8 18 8.
Protons neutrons and electrons.
40 Zr Zirconium 91224 2 8 18 10 2.
Deep inside stars the pressure is so high that hydrogen atoms are converted to helium atomsThis conversion is called fusion and it releases heat and energy that we see as sunlight.
38 Sr Strontium 87620 2 8 18 8 2.
Refer to the table below and work out the number of Electrons in various elements.
Pentavalent elements are those elements which have five electrons in their outer shell.
In chemistry and atomic physics an electron shell may be thought of as an orbit followed by electrons around an atoms nucleusThe closest shell to the nucleus is called the 1 shell also called the K shell followed by the 2 shell or L shell then the 3 shell or M shell and so on farther and farther from the nucleusThe shells correspond to the principal quantum numbers n.
Valence Electrons Chart - Valence Electrons of all the elements in table chart.
Photocatalysis results from the common effect of holes and electrons.
5A or VA along with nonmetal phosphorus P the metalloids arsenic As and antimony Sb and the metal bismuth Bi.
The total number of electrons present in the valence shell of an atom are called valence electrons.
Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver.
42 Mo Molybdenum 9595 2 8 18 13 1.
Elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table.
Arsenic occurs in many minerals usually in combination with sulfur and metals but also as a pure elemental crystalArsenic is a metalloidIt has various allotropes but only the gray form which has a metallic appearance is important to industry.
The isotope is defined by the number of neutrons in an atom which might be equal to the number of protonsor not.
As oxygen belongs to group 16 6A or VIA along with sulfur S arsenic As and selenium Se tellurium Te polonium Po and livermorium Lv.
The number of electrons and holes in an intrinsic semiconductor are equal.
This leaves a low number of electrons available for conduction.
So for our example we would say that sodium has 2 electrons in the 1s orbital plus 2 electrons in the 2s orbital plus 6 electrons in the 2p orbital plus 1 electron in the 3s orbital.
It provides millions of free electrons for conduction.
Metal oxide materials such as TiO 2 WO 3 ZnO and many other oxides belong to n-type semiconductors.
Since an extremely small amount of arsenic impurity has a large number of atoms.
Stars and Planets Hydrogen is found mostly in stars and gas giant planets.
Generally elements in Groups 1 2 and 13 to 17 tend to react to form a closed shell with a noble gas electron configuration ending in ns2 np6.
Less than 002 of the arsenic was excreted in the urine and 03 was detected in the.
The most reactive metals are those from Groups 1 and 2.

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen

Krypton Facts Information Pictures Encyclopedia Com Articles About Krypton Biology Facts Science Chemistry Electrons

Science Coverage Valency Of Cesium How Many Valence Electrons Doe In 2021 Electron Configuration Electrons Noble Gas

Sezen Kocabiyik Et 90 Autres Utilisateurs Ont Enregistre 81 De Vos Epingles Chemistry Lessons Chemistry Classroom Electron Configuration

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Atom Model

Oxygen Electron Configuration How To Write The Electron Configuration For Oxygen O In 2021 Electron Configuration Electrons Oxygen

Science Coverage Valency Of Sodium How Many Valence Electrons Doe Electrons Electron Configuration Sodium

Electron Configuration Of Every Element In The Periodic Table Biochemhelp Chemistry Lessons Chemistry Classroom Organic Chemistry Study

Periodic Table Song X2f Periodic Table Of Elements X2f Metalloids Youtube Songs Stem For Kids Kids Learning

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

Beautiful Crunchies A Sample Of The Element Arsenic In The Periodic Table Arsenic Periodic Table Element

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons










No comments:
Post a Comment