
The Atom Is Defined As The Smallest Particle Of An Element That Still Has Properties Of That Element But Where Did This Idea Come From Ppt Download

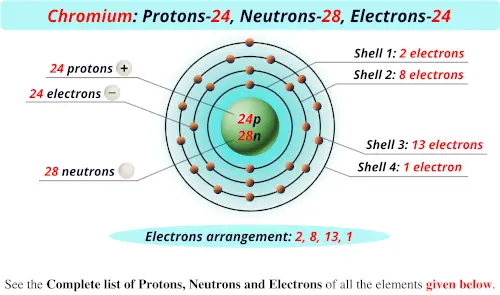
Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

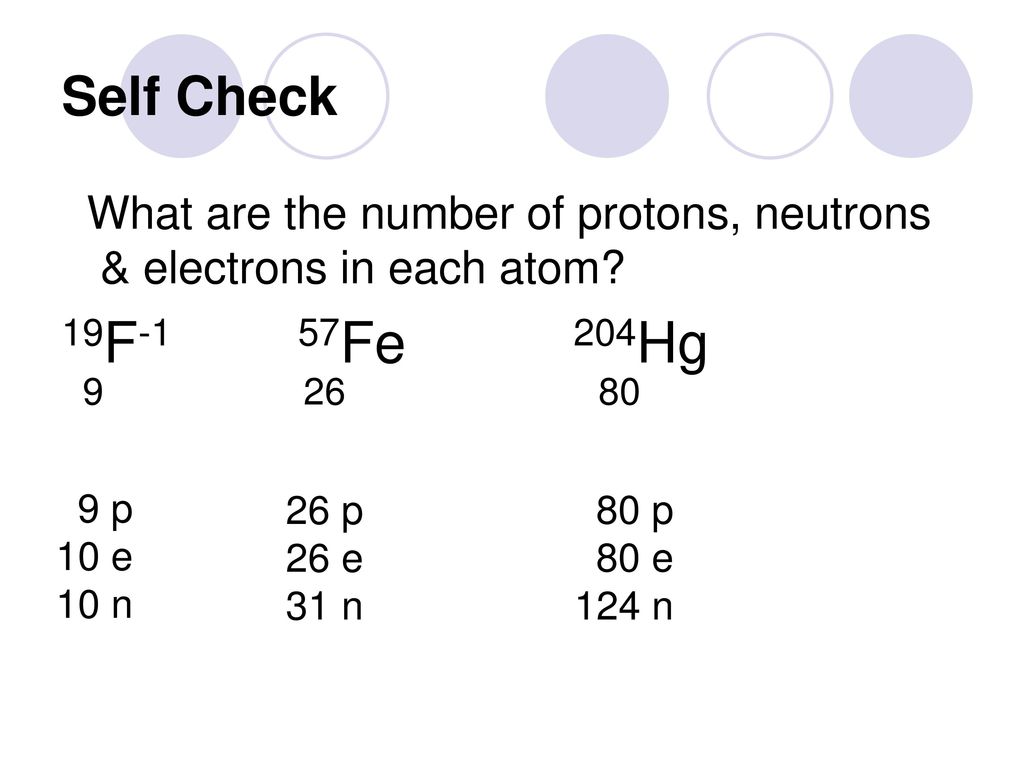
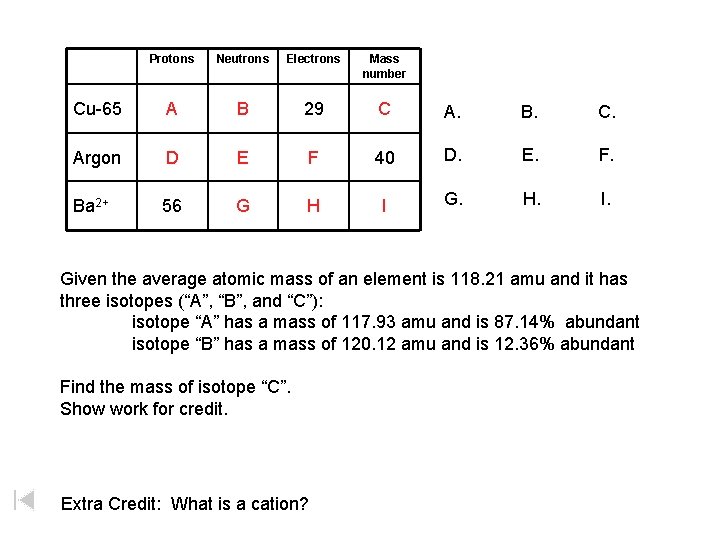
Homework Problems Chapter 2 Homework Problems 1 4 19 24 28 Give The Number Of Protons Neutrons And Electrons 30 38 41 44 46 54 64 66 Ppt Download

The Atom Is Defined As The Smallest Particle Of An Element That Still Has Properties Of That Element But Where Did This Idea Come From Ppt Download

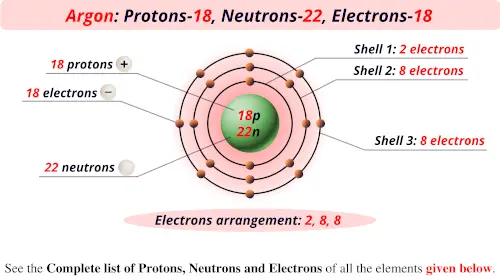
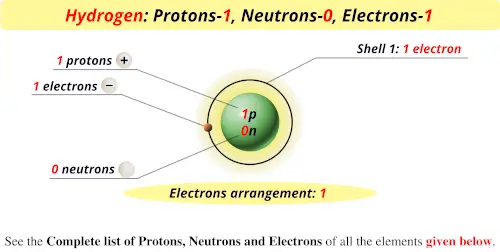
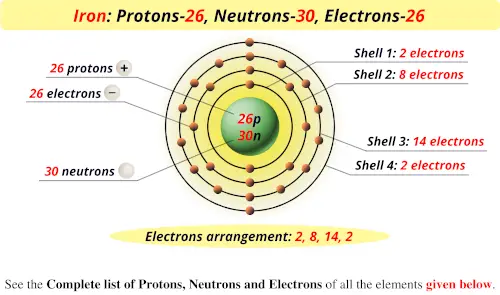
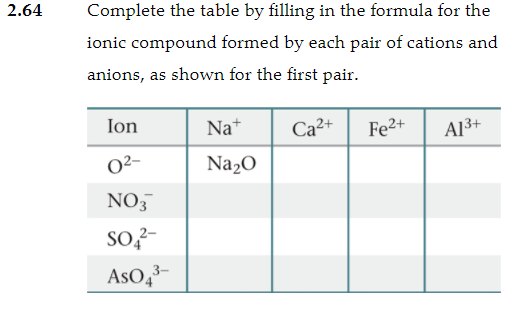
Chapter 2 Atoms Molecules And Ions 2 Atoms Molecules And Ions Page 26 13 Complete The Following Chart In Order From Left To Right Ion Mass Number Protons Neutrons Electrons Pdf Document













No comments:
Post a Comment