
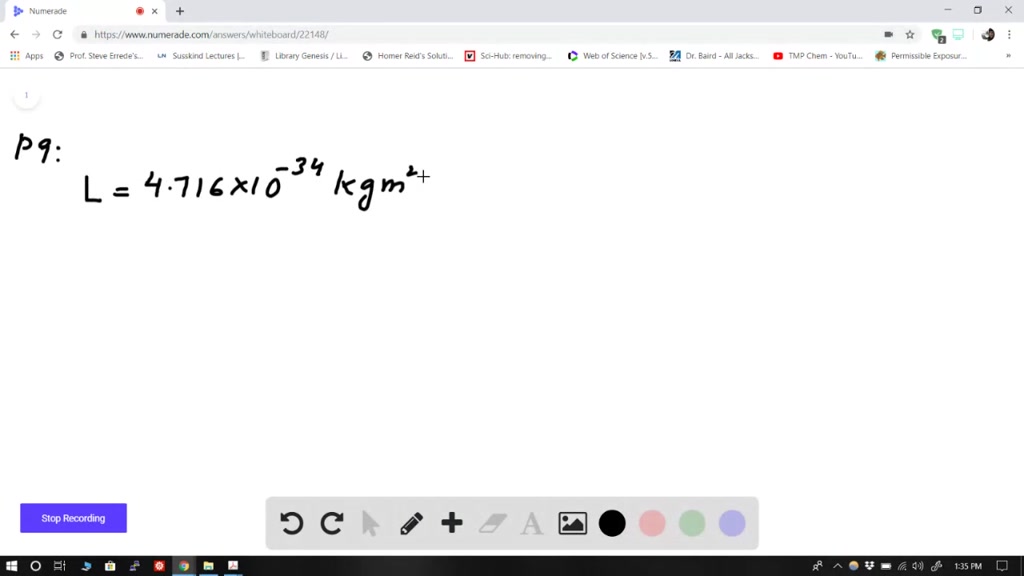
Solved The Orbital Angular Momentum Of An Electron Has A Magnitude Of 4 716 Times 10 34 Kg Cdot M 2 S What Is The

The Magnitude Of Angular Momentum Orbital Radius And Time Period Of Revolution Of An Electron In A Hydrogen Atom Corresponding To The Quantum Number N Are L R And T Respectively

What Is The Angular Momentum Of An Electron In Bohr S Hydrogen Atom Whose Energy Is 0 544 Ev Youtube

Yuri Kovalenok On Instagram Friends A Few Useful Notes On Quantum Physics A Video With My Favorite Quantum In 2021 Physics And Mathematics Physics Quantum Physics

Consider Bohr S Theory For Hydrogen Atom The Magnitude Of Orbit Angular Momentum Orbit Radius And Velocity Of The Electron In Nth Energy State In A Hydrogen Atom Are L R

Bohr Model Of Atom Results For Hydrogen Atom Or Ion Containing 1 Electron Bohr Model Hydrogen Atom Energy Level
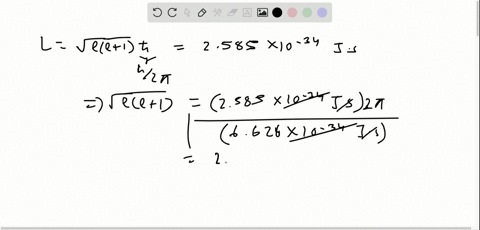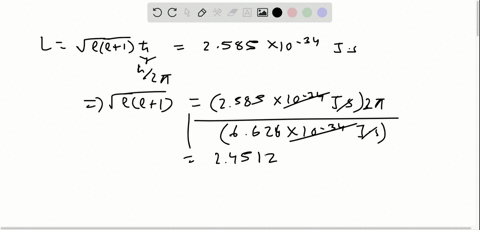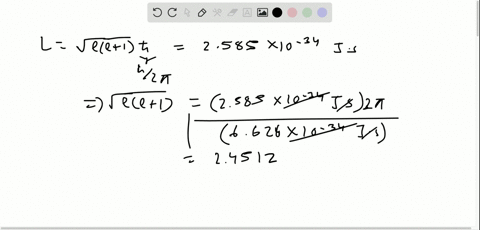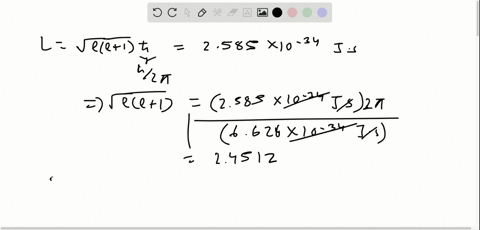
Solved The Orbital Angular Momentum Of An Electron Has A Magnitude Of 4 716 Times 10 34 Kg Cdot M 2 S What Is The
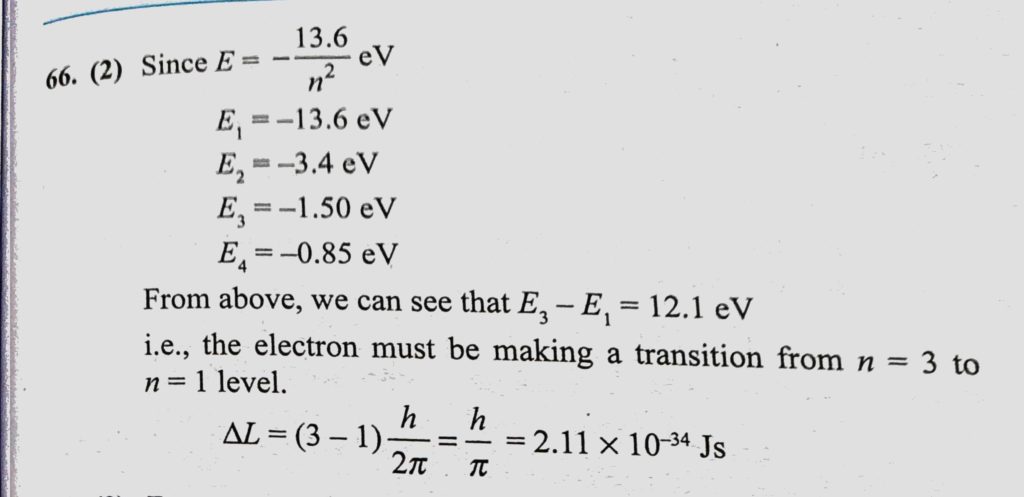
If A Hydrogen Atom Emits A Photon Of Energy 12 1 Ev Its Orbital Angular Momentum Changes By Delta L Then Delta L Equals Sahay Lms

The Magnitude Of The Spin Angular Momentum Corresponding To An Electron In Balmer Transition Inside Youtube
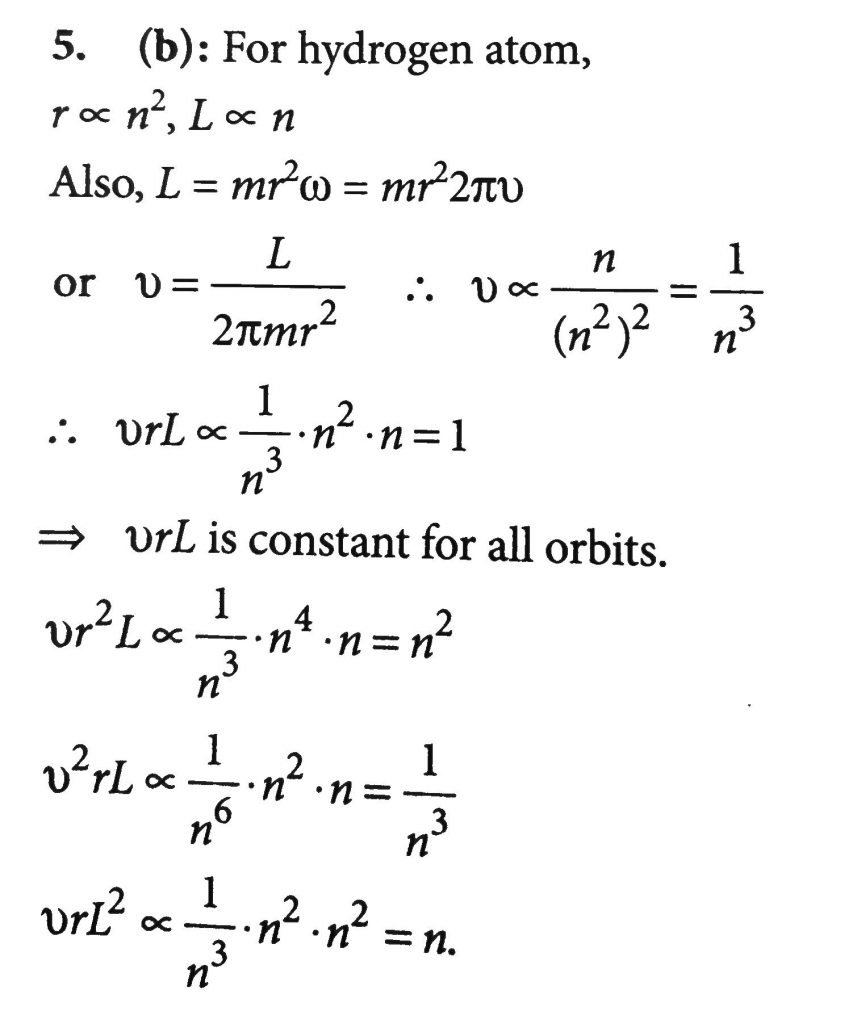
The Magnitude Of Angular Momentum Orbit Radius And Frequency Of Revolution Of Electron In Hydrogen Atom Corresponding To Quantum Number N Are L R And V Respectively Then According To Bohr S Theory




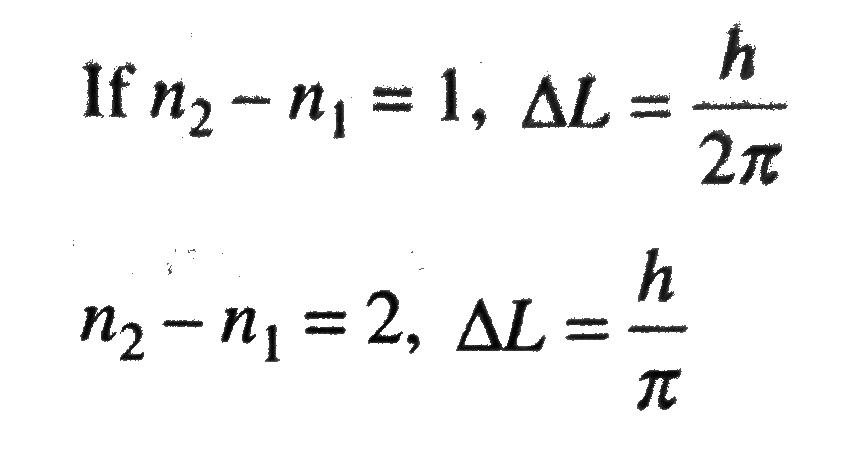




No comments:
Post a Comment