The electron configuration for the first 10 elements. More detailed versions of the periodic table you can find an excellent example here often show the electron configuration as a comma-separated list of values showing the number of electrons in each shellFor example silicon Si would have the electron configuration 2 8 4Electron shells 1n and 2n are full containing two and eight electrons respectively while electron shell 3n contains.
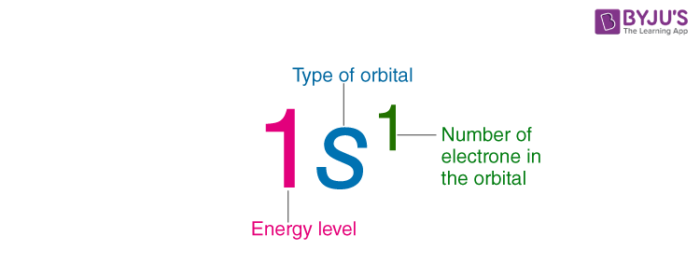
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.

Abbreviated electron configuration for helium.
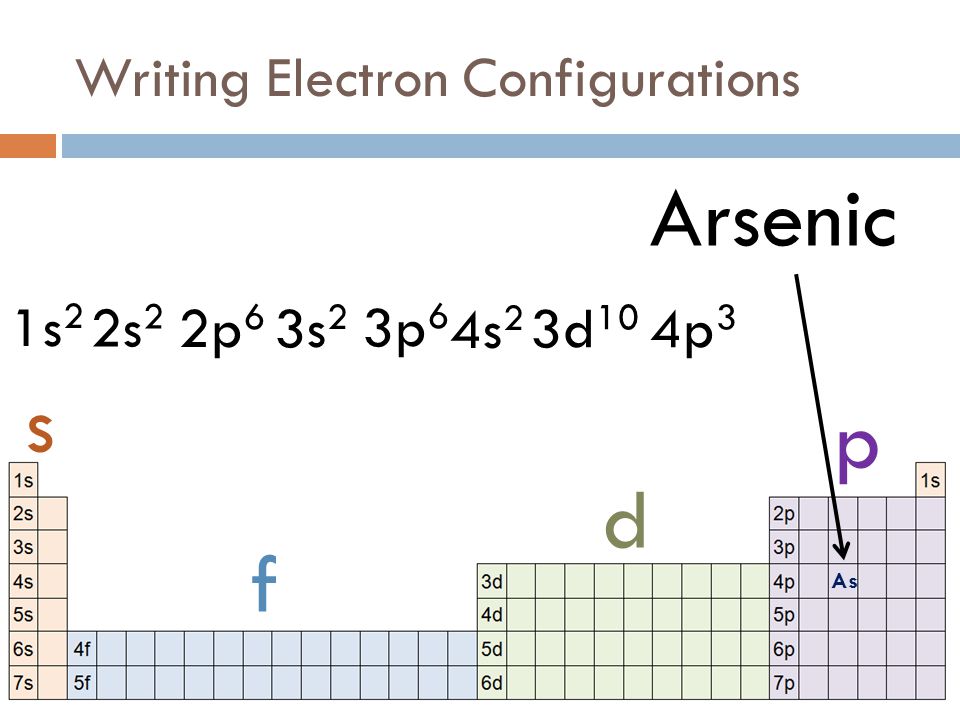
Therefore the general electron configuration for an element in the p-block is ns 2 np 1-6.
Electron Configurations are useful for.
For example the electron configuration of elements in Group 13 is ns 2 np 1 the configuration of elements in Group 15 is ns 2 np 3 and so on.
Therefore after spin integration spatial integrals in.
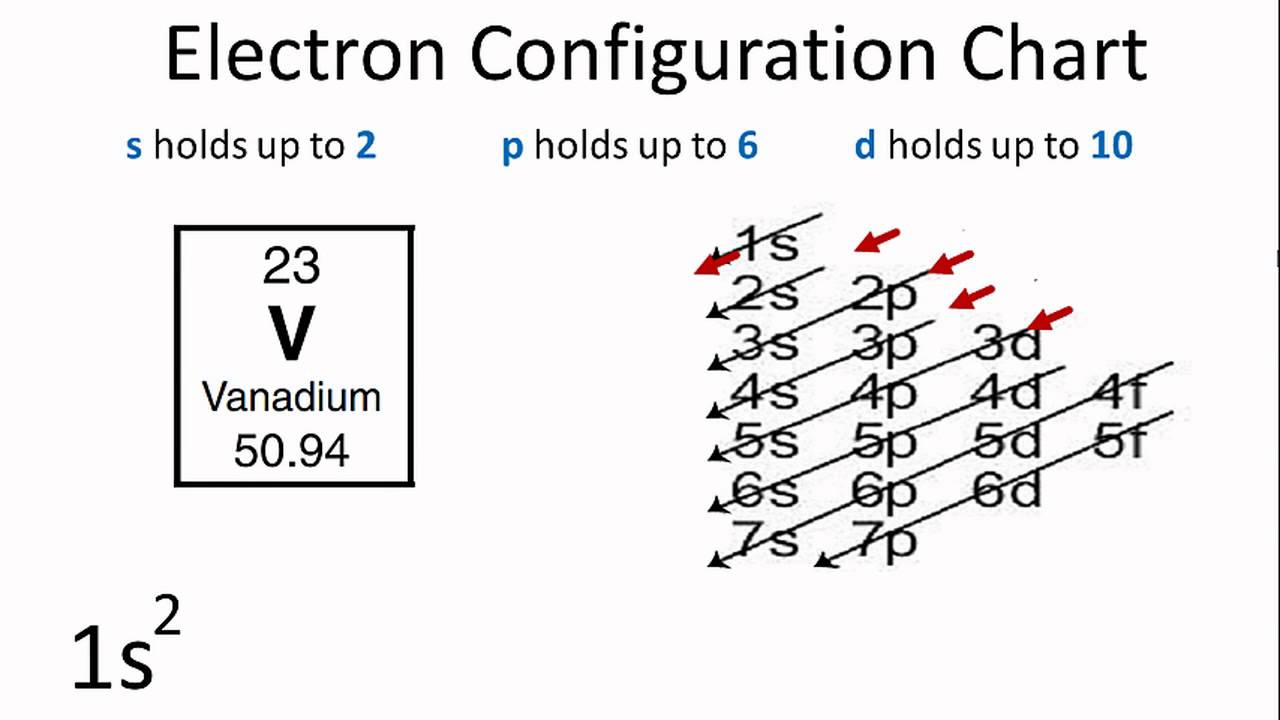
Iron Fe - Iron is represented as Fe and has an atomic number of 26.
Know the physical and chemical properties density boiling and melting point along with the uses of Iron on BYJUS.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
As a general rule a main-group element except hydrogen or helium tends to react to form a s 2 p 6 electron configurationThis tendency is called the octet rule because each bonded atom has 8 valence electrons including shared electronsSimilarly a transition metal tends to react to form a d 10 s 2 p 6 electron configurationThis tendency is called the 18-electron rule because each bonded.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass.
Electron application boilerplate based on React React Router Webpack React Hot Loader for rapid application development.
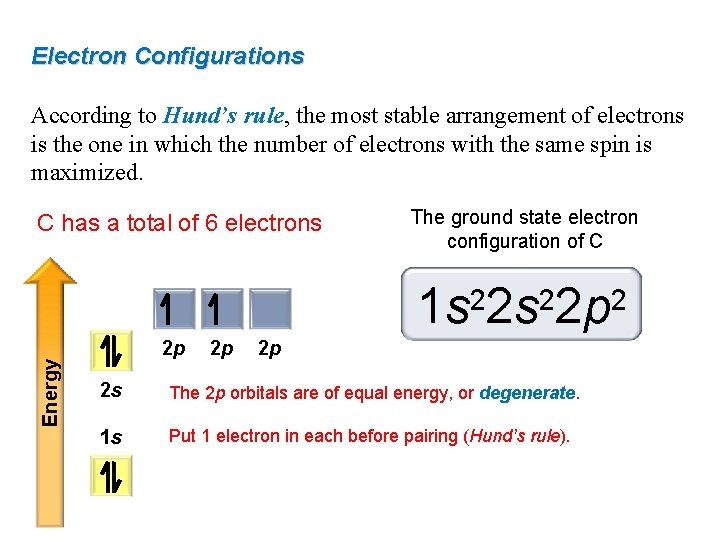
Draw a cartoon energy level diagram with lines for the valence atomic energy levels orbitals of each atom.
First ionisation energy The minimum energy required to remove an electron from a neutral atom in its.
Electronegativity Pauling scale The tendency of an atom to attract electrons towards itself expressed on a relative scale.
Helium atom example Spatial part is the same.
This shared electron density lies directly.
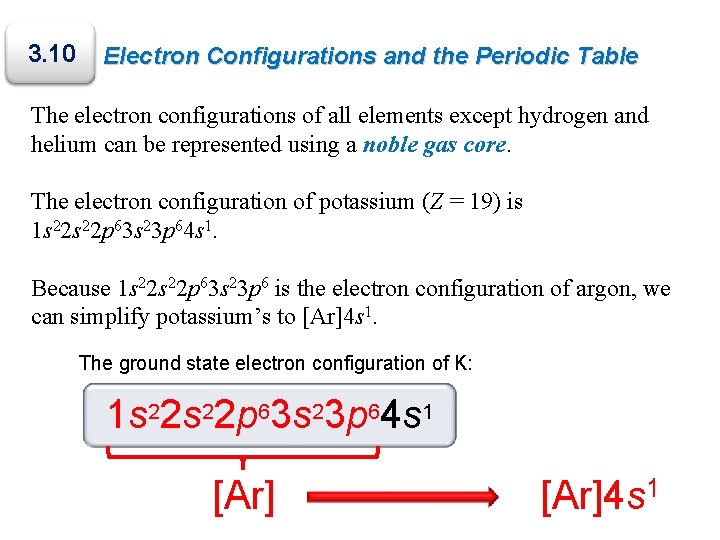
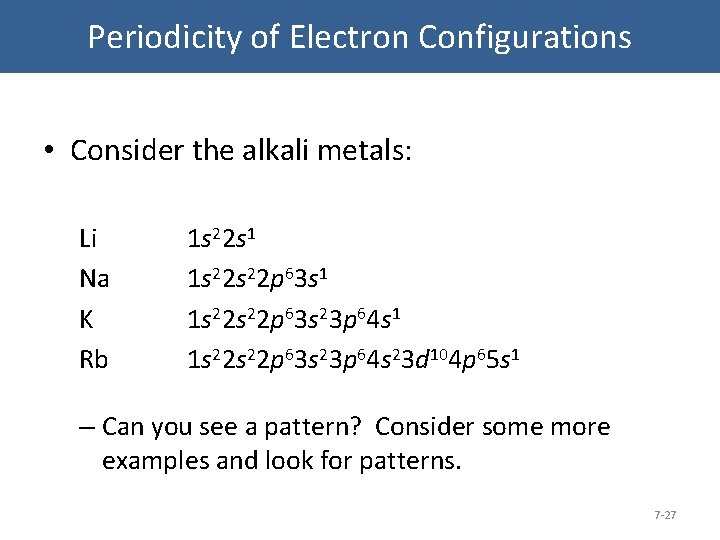
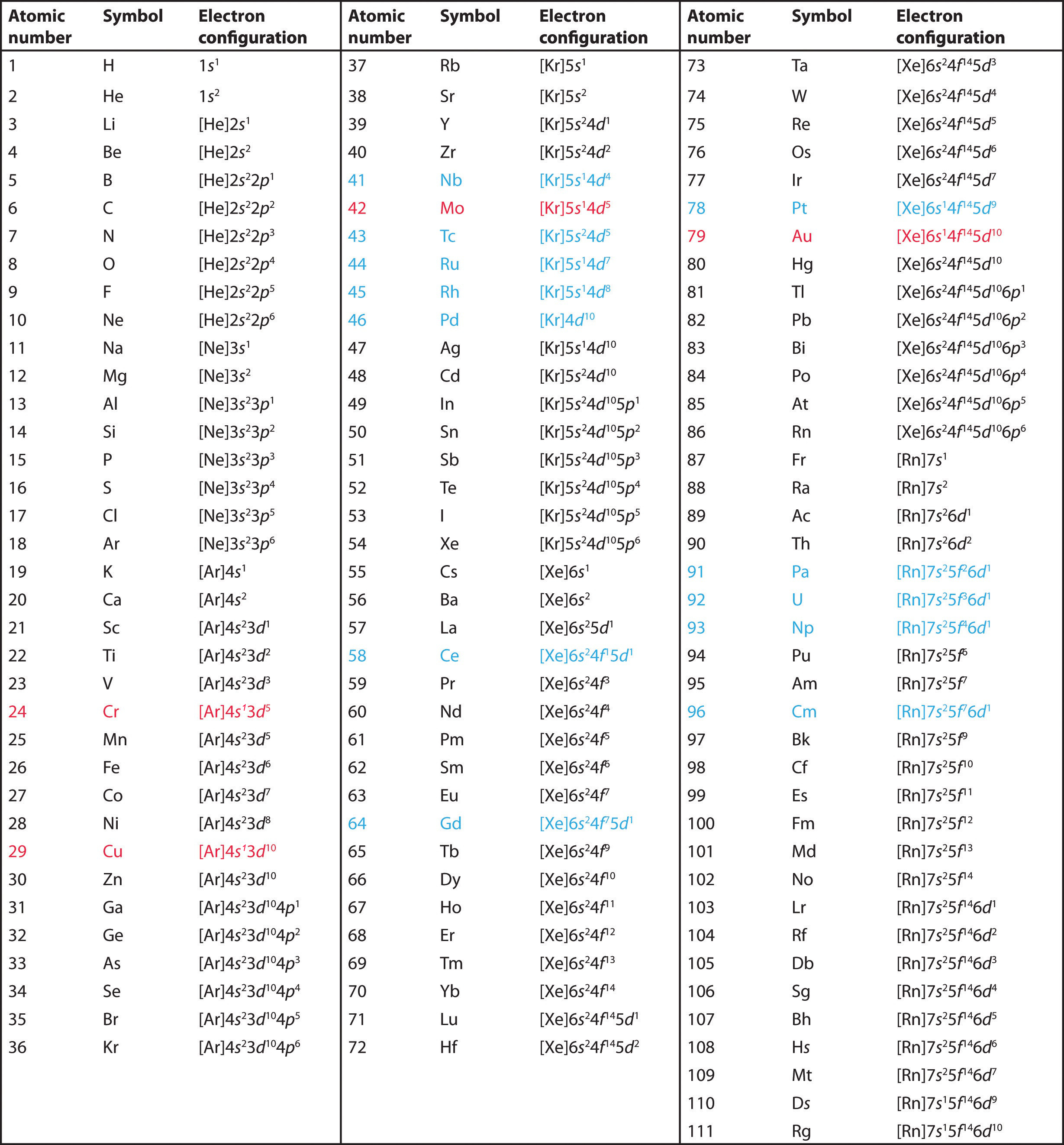
Similarly the abbreviated configuration of lithium can be represented as He2s 1 where He represents the configuration of the helium atom which is identical to that of the filled inner shell of lithium.
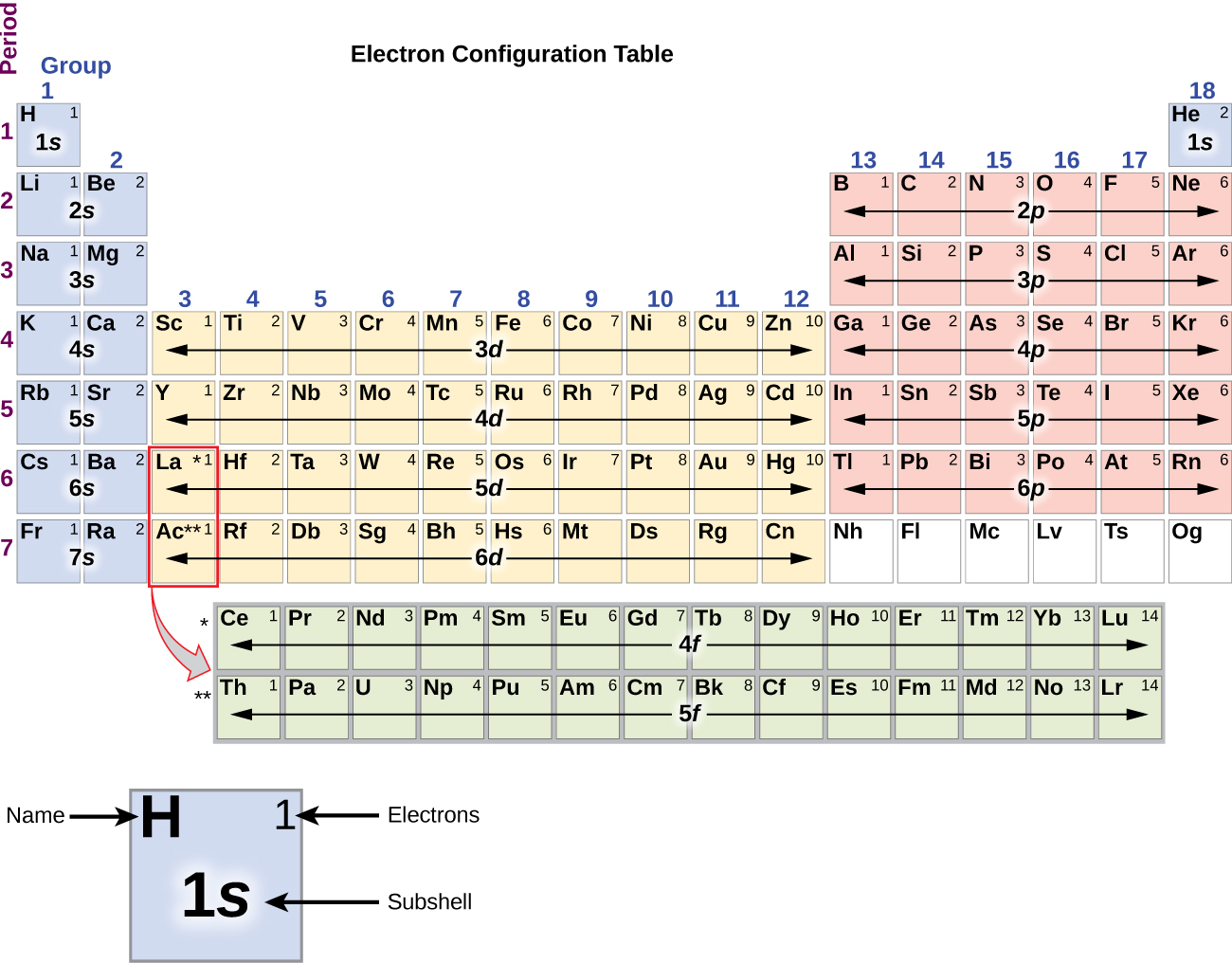
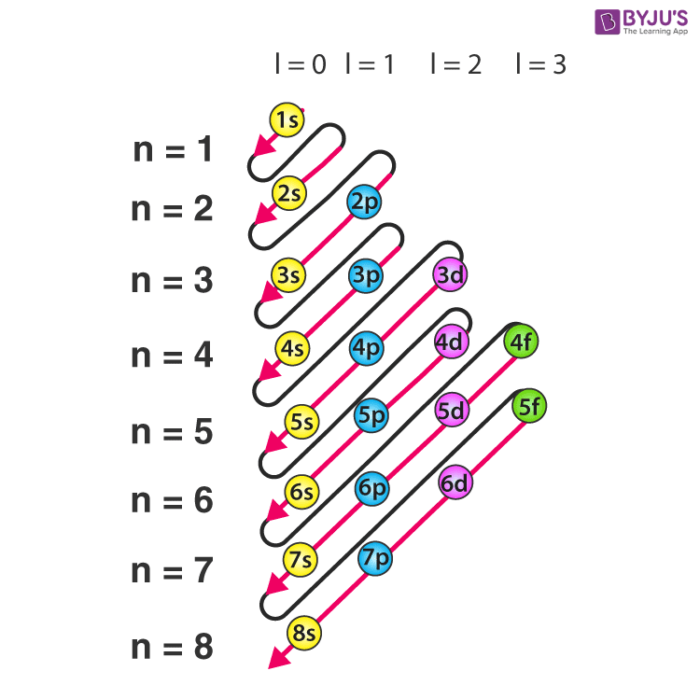
An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons may occupy.
Determining the valency of an element.
The elements of Group 18 helium neon argon krypton xenon and radon are called the noble gases.
The most common compound is sodium chloride.
The helium Noble gas configuration by sharing electrons and form a molecule.
This gives you the total number of electrons you will have to distribute among the molecular orbitals you form.
As befits an element that flies under the chemical and physical radar unless provoked it.
Invalid packet ctb0a no signature.
For example consider B 2 each atom has an electron configuration of He2s 2 2p which has a total of 6 valence electrons.
Argon abbreviated Ar is element number 18 on the periodic table making it the third-lightest of the six noble gases behind helium atomic number 2 and neon number 10.
Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.
Or abbreviated DIE is a program for determining types of files.
Sometimes abbreviated au or E h H atom energy in the Born-Oppenheimer approximation use electron mass not.
Sodium is the sixth most common element on Earth and makes up 26 of the Earths crust.
Therefore the abbreviated electron configuration of sodium is Ne3s 1 the electron configuration of neon is 1s 2 2s 2 2p 6 which can be abbreviated to He2s 2 2p 6.
Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed.
Writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium.
Iron atomic mass is 55845 u.
The configuration app for the AudioMoth acoustic monitoring device.
Each hydrogen atom brings a single electron in its 1s atomic orbital to share electron density thus acquiring two electrons in its valence shell.
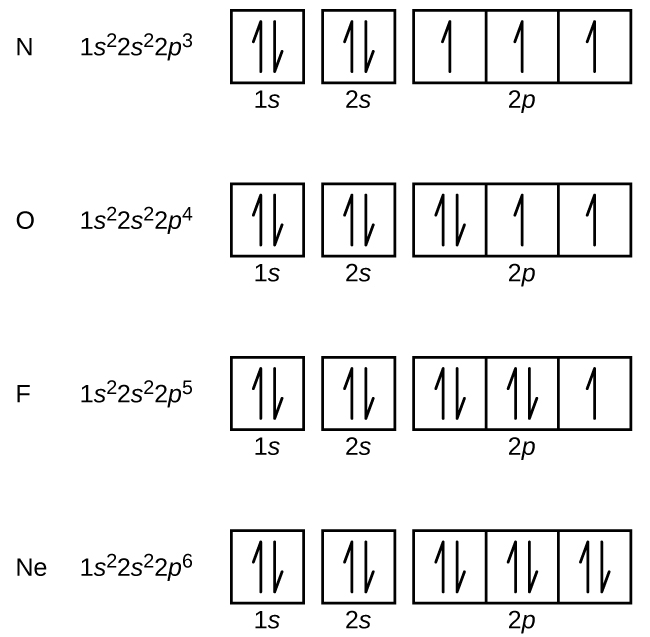
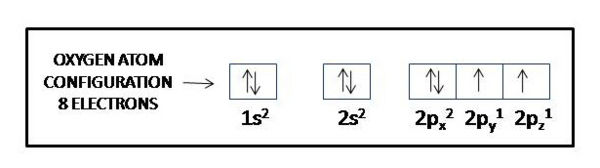
H 1s1 He 1s2 Li 1s2 2s1 Be 1s2 2s2 B 1s2 2s2 2p1 C 1s2 2s2 2p2 N 1s2 2s2 2p3 O 1s2 2s2 2p4 F 1s2 2s2 2p5.
We can use Hartree-Fock theory to understand this As an example.
This very soluble salt has been leached into the oceans over the lifetime of the planet but many salt beds or lakes are found where ancient seas have evaporated.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.

Predict The Ground State Electron Configuration Of The Following Ions Write The Answer In Brainly Com











No comments:
Post a Comment